Answer: The number of carbon atoms in 200 molecules of given sample is 600 atoms.
Step-by-step explanation:
We are given a chemical compound having chemical formula
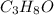
In 1 molecule of
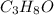 , 3 atoms of carbon, 8 atoms of hydrogen and 1 atom of oxygen are present.
, 3 atoms of carbon, 8 atoms of hydrogen and 1 atom of oxygen are present.
We need to calculate the number of carbon atoms in 200 molecules of the sample.
So, in 200 molecules of
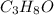 , the number of carbon atoms are =
, the number of carbon atoms are =
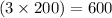 atoms.
atoms.
Hence, the number of carbon atoms in 200 molecules of given sample is 600 atoms.