Answer:
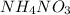 has pH<7
has pH<7
Step-by-step explanation:
pH of salt depends on species generated through hydrolysis of the given salt.
Hydrolysis equilibrium of
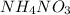 is given below:
is given below:
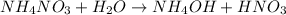
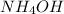 is a weak base and
is a weak base and
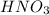 is a strong acid. So species obtained through hydrolysis of
is a strong acid. So species obtained through hydrolysis of
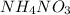 is a mixture of strong acid and weak base.
is a mixture of strong acid and weak base.
Hence the the salt solution will be acidic in nature. That means pH of solution is less than 7.