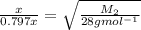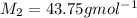Answer: Molar mass of unknown gas=43.75
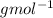
Explanation: Graham's Law states that the rate of effusion of gas is inversely proportional to the square root of their atomic masses.
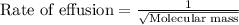
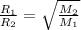
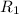 = rate of effusion of nitrogen = x
= rate of effusion of nitrogen = x
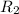 = rate of effusion of unknown gas = 0.797x
= rate of effusion of unknown gas = 0.797x
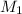 = Molecular mass of nitrogen
= Molecular mass of nitrogen
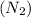 = 28g
= 28g
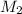 = Molecular mass of unknown gas
= Molecular mass of unknown gas