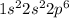Answer: The electron configuration for an electrically neutral atom of neon is
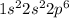
Step-by-step explanation:
Electronic configuration is defined as the representation of electrons around the nucleus of an atom.
Number of electrons in an atom is determined by the atomic number of that atom.
Neon is the 10th element of the periodic table. The number of electrons present in the neutral atom of neon are 10.
The electronic configuration of neutral neon atom is
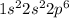
Hence, the electron configuration for an electrically neutral atom of neon is