Step-by-step explanation:
The given data is as follows.
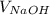 = 26.25 ml,
= 26.25 ml,
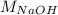 = 0.1850 m
= 0.1850 m
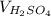 = 25.00 ml,
= 25.00 ml,
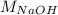 = ?
= ?
It is known that normality is n times molarity where "n" signifies the number of hydrogen or hydroxide ions.
Therefore, normality of NaOH is calculated as follows.
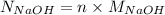
=
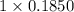
= 0.1850 N
Normality of
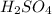 is calculated as follows.
is calculated as follows.
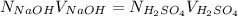
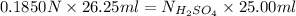
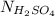 = 0.194 N
= 0.194 N
Hence, molarity of
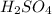 will be as follows.
will be as follows.
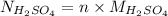
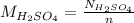
=
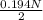
= 0.097 M
Thus, we can conclude that molarity of the acid solution is 0.097 M.