Answer : The entropy change of the system is, 19.5 J/K
Solution :
Formula used :
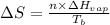
or,
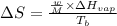
where,
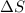 = entropy change of the system = ?
= entropy change of the system = ?
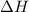 = enthalpy of vaporization = 30.7 kJ/mole
= enthalpy of vaporization = 30.7 kJ/mole
n = number of moles of benzene
w = mass of benzene = 17.5 g
M = molar mass of benzene = 78 g/mole
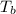 = normal boiling point of benzene =
= normal boiling point of benzene =
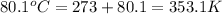
Now put all the given values in the above formula, we get the entropy change of the system.
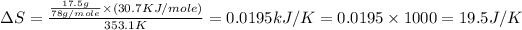
Therefore, the entropy change of the system is, 19.5 J/K