Answer:
(a)
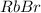 - rubidium bromide.
- rubidium bromide.
(b)
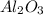 - aluminium oxide.
- aluminium oxide.
(c)
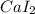 - calcium iodide.
- calcium iodide.
Step-by-step explanation:
Hello,
(a) In this case, the elements having 37 and 35 as the oxidation states are rubidium and bromine respectively, in such a way the formed compound, due their gatherable oxidation states (+1 and -1 respectively) is the following binary salt:
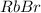 - rubidium bromide.
- rubidium bromide.
(b) In this case, the elements having 8 and 13 as the oxidation states are aluminium and oxygen respectively, in such a way the formed compound, due their gatherable oxidation states (+3 and -2 respectively) is the following basic oxide:
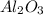 - aluminium oxide.
- aluminium oxide.
(c) In this case, the elements having 20 and 53 as the oxidation states are calcium and iodine respectively, in such a way the formed compound, due their gatherable oxidation states (+2 and -1 respectively) is the following binary salt:
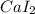 - Calcium iodide.
- Calcium iodide.
Best regards.