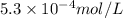Answer: The solubility of
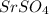 is
is
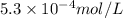
Step-by-step explanation:
It is given that
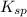 of strontium sulfate is
of strontium sulfate is
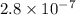
The balanced equilibrium reaction for ionization of
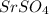 is given by:
is given by:
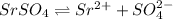
At equilibrium: s s
The equation to calculate solubility constant is given as:
![K_(sp)=[Sr^(2+)][SO^(2-)_4]](https://img.qammunity.org/2018/formulas/chemistry/college/2b8lolvsvj99rg1ffiiwebpay4f89pyqee.png)
Now put the given values in above equation, we get:
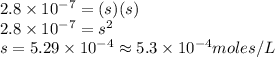
Therefore, the solubility of
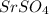 is
is