Answer:
7.875 gram is the actual yield of product obtained.
Step-by-step explanation:
We are given:
Theoretical yield of reaction = 10.5 g
Experimental or actual yield = x
Percentage yield of the product = 75.5 %
The formula we used to determine percentage yield is:
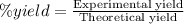
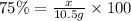
x = 7.875 g
7.875 gram is the actual yield of product obtained.