Step-by-step explanation:
Acetic acid
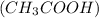 is a weak acid therefore, when dissolved in a liquid it slightly dissociates into its ions. That is, there will be very less number of hydrogen ions given by acetic acid upon dissociation.
is a weak acid therefore, when dissolved in a liquid it slightly dissociates into its ions. That is, there will be very less number of hydrogen ions given by acetic acid upon dissociation.
Therefore, few safety concerns are required while handling or using acetic acid.
Whereas hydrochloric acid (HCl) is a strong acid and when dissolved in a liquid it completely dissociates into its ions. That is, there will be more number of hydrogen ions given by HCl upon dissociation.
Hence, these hydrogen ions will readily reaction with other ions or materials hence, it will be dangerous in nature.
Thus, more safety concerns are required while handling hydrochloric acid like safety googles and gloves should be worn before using it.