Answer:
The concentration of hydronium ions in solution is
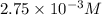 .
.
Step-by-step explanation:
The pH of the solution is defined as negative logarithm of hydronium ions concentration in a solution. Mathematically written as:
![pH=-\log[H_3O^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/orq7jc6zru7gow6jwtng30eu5b9rtp83tt.png)
Given, the pH of the solution = 2.56
![2.56=-\log[H_3O^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/g9js1spyf2a0ljbofjhambpvsjegacjj10.png)
![[H_3O^+]=0.002754 M=2.75* 10^(-3) M](https://img.qammunity.org/2018/formulas/chemistry/high-school/s7he0hcp2s5y1pin4yxnd9vfo6eewmsqn2.png)
The concentration of hydronium ions in solution is
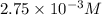 .
.