I'm assuming that by "miles" you mean moles.
If O2 is the excess reactant, that means Fe is the limiting reactant. That means that the amount of product being formed depends on the amount of Fe reactant present. To calculate the moles of Fe2O3 formed, start with the given 6.4 moles of Fe and use the mole to mole ratio given by the reaction as shown below:
6.4 mol Fe x
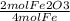
=
3.2 mol Fe2O3