Answer: Option (A) is the correct answer.
Step-by-step explanation:
An alpha particle is a helium atom that is represented as
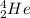 .
.
Therefore, when an alpha decay occurs if polonium atom has 84 protons, 124 mass, and 84 electrons. Then the reaction will be as follows.
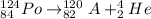
An element with atomic number 82 is lead.
Thus, we can conclude that when a polonium atom has 84 protons, 124 mass, and 84 electrons then it will change to a lead atom with 82 protons, 122 mass, and 82 electrons during alpha decay.