Answer: Average distance between gas molecules become double.
Explanation :
Average distance or mean free path of a gas molecule is given as :
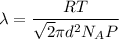
Where,
R is gas constant
T is temperature
d is the radius of the gas molecule
P is the pressure
We know that all values except P are constant.
Also,
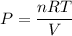
So,
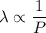
So,
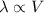
When a container with a specific volume “V” changes to “2V. Then the average distance between the gas molecules become double