Answer:
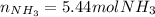
Step-by-step explanation:
Hello,
At first, let the undergoing chemical reaction to be rewritten:

Based on it, we apply the stoichiometric relationship between ammonia and fluorine to determine the completely reacting moles of ammonia, taking into account the 2 to 5 mole ratio between ammonia and fluorine respectively as shown below:
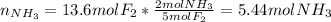 }}
}}
Best regards.