Answer:
0.221 g of
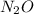 have 0.010 mol of N
have 0.010 mol of N
Step-by-step explanation:
Mass of
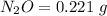
Step 1
Calculation of moles of
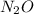
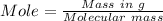
Molecular mass of
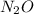 = 14 × 2 + 16 = 44
= 14 × 2 + 16 = 44
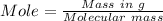
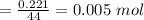
Calculation of moles of N
1 mol of
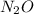 has 2 moles of N
has 2 moles of N
0.005 mol of
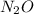 will have
will have
= 0.005 × 2 = 0.010 mol of N
So, 0.221 g of
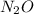 have 0.010 mol of N
have 0.010 mol of N