Step-by-step explanation:
A compound formed by transfer of electrons from one atom to another is known as ionic compound. And, bond formed due to transfer to this type of transfer is known as ionic bond.
Therefore,
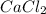 is an ionic compound as it is formed by transfer of electrons from calcium to chlorine atoms.
is an ionic compound as it is formed by transfer of electrons from calcium to chlorine atoms.
This compound is formed by chemical combination of one calcium and two chlorine atoms.
This means basically,
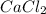 is made up of two elements which are calcium and chlorine.
is made up of two elements which are calcium and chlorine.