Answer:
We have to heat the mixture at 100 °C or above to remove water to get pure sample.
Step-by-step explanation:
The reaction between given HBr and AgOH will be:
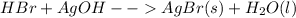
Now after this reaction we are getting AgBr and water as products.
To get pure HBr we have o remove water. Water can be easily removed by heating the sample at or above its boiling point, 100°C.
The steps will be
a) Filter the precipitate of HBr.
b) Dry the sample by heating it in hot air oven at or above °C.