Answer:
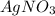 and
and
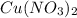
Step-by-step explanation:
Hello,
Based on the chemical reaction, both silver nitrate and cupric nitrate are dissolved in water due to their aqueous aggregation state during the chemical reaction. This is owing to their relatively high solubility in water unlike the pure both copper and the silver since they are metals that of course do not dissolve into water by cause of their metallic nature.
Best regards.