Answer: The mass of the solid will be 2.69 grams.
Explanation:
Density is defined as the mass contained per unit volume.
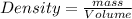
Given :
Density of solid =
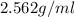
Mass of solid = ?grams
Volume of water displaced = 1.05 ml
Putting in the values we get:
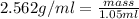

Thus the mass of solid is 2.69 grams.