Answer: The water becomes warmer.
Explanation:
Average kinetic energy is defined as the average of the kinetic energies of all the particles present in a system. The molecules start moving more randomly. It is determined by the equation:
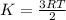
From above, it is visible that kinetic energy is directly related to the temperature of the system. So, if the average molecular kinetic energy increases, the temperature of the water must have increased and thus the water will become warmer.