Answer: The expression for the equilibrium of the given reaction is
![[A}^a[B]^b=[C]^c[D]^d](https://img.qammunity.org/2018/formulas/chemistry/high-school/l4a5joe0dqearu7zu608bkmer3byj8sfpn.png)
Step-by-step explanation:
Equilibrium is defined as the condition when ratio of the concentration of products and the reactants does not vary. It is also the state when the rate of forward reaction is equal to the rate of backward reaction.
To write the rate of the reaction, we use Law of mass action which states that the rate of the reaction is expresses in the terms of molar concentration of the reactants with each raised to power their stoichiometric coefficients.
For the given reaction:
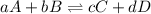
Rate of forward reaction:
![[A}^a[B]^b](https://img.qammunity.org/2018/formulas/chemistry/high-school/86mnjne0jgo91xx271mexhk2agi6mk9p5g.png)
Rate of backward reaction:
![[C]^c[D]^d](https://img.qammunity.org/2018/formulas/chemistry/high-school/73m8hotkkjevcdpei3wckynpte2o12zky0.png)
As, rate of forward reaction = rate of backward reaction.
So, the expression for the equilibrium of the given reaction is
![[A}^a[B]^b=[C]^c[D]^d](https://img.qammunity.org/2018/formulas/chemistry/high-school/l4a5joe0dqearu7zu608bkmer3byj8sfpn.png)