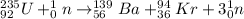Answer:
The radioisotope produces daughter atoms or another atoms of elements and also releases some characteristic radiation on nuclear decay.
Step-by-step explanation:
Nuclear decay is the process in which a radioactive isotope which is quite unstable undergoes fission reaction to produce daughter atoms or another atoms of elements and also releases some characteristic radiation. These can be alpha, beta or gamma particles.
For example, the nuclear decay reaction of Uranium-235 isotope is shown below in which it forms Barium and Krypton atoms with the release of neutrons.