Answer:
0.30 M is the total concentration of ions in a 0.10 M solution of barium chloride.
Step-by-step explanation:
Concentration of solution of barium chloride = 0.10 M = 0.10 mol/L
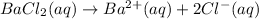
1 Mole of barium chloride gives 1 mole of barium ions and 2 moles of chloride ions.
Concentration of barium ions in solution = [x]
![[x]=1* 0.10 mol/L=0.10 mol/L](https://img.qammunity.org/2018/formulas/chemistry/college/w0z6st79mcfqffx359tl3ym0yugijkhiyu.png)
Concentration of chloride ions in solution = [y]
![[y]=2* 0.10 mol/L=0.20 mol/L](https://img.qammunity.org/2018/formulas/chemistry/college/xgfxftqkngxewcsgbr5eb8h73ytgjhr8o1.png)
Total concentration of ions in the solution : [x]+[y]
0.10 mol/L + 0.20 mol/L = 0.30 mol/L
0.30 M is the total concentration of ions in a 0.10 M solution of barium chloride.