Answer: Option (D) is the correct answer.
Step-by-step explanation:
It is given that pressure is constant and the given data is as follows.
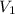 = 492 mL,
= 492 mL,
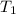 = 31 + 273 = 304 K
= 31 + 273 = 304 K
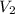 = 876 mL,
= 876 mL,
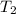 = ?
= ?
Therefore, calculate the temperature
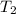 as follows.
as follows.
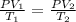
Since pressure is constant so, value of P = 1.
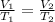
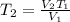
=
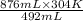
= 541.268 K
= 541 K (approx)
Thus, we can conclude that value of
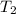 is 541 K.
is 541 K.