Answer: Zinc element was the electron donor
Step-by-step explanation:
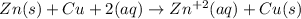
In the given reaction, the zinc metal is getting oxidized to zinc ion by loosing 2 electrons and copper ion are getting reduced to copper by gaining 2 electrons.

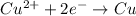
So, in the given reaction zinc is an element which donates its electrons.Hence, electron donor was the zinc element.