Answer:
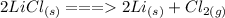
Step-by-step explanation:
The state symbols are important when writing balanced chemical equations, especially when they have been specified in the question. Chlorine is a diatomic gas when it is an element so that should not be forgotten when chlorine is isolated.