Answer: Option (B) is the correct answer.
Explanation:
When more effectively an acid is able to dissociate completely and give a proton or
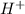 ion then it is known as acid strength.
ion then it is known as acid strength.
For example, HCl is a strong acid and thus completely dissociates to give proton.
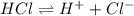
Whereas
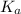 is known as the acid dissociation constant. More is the value of
is known as the acid dissociation constant. More is the value of
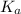 more will be the acidic strength of acid.
more will be the acidic strength of acid.
Thus, we can conclude that when the
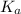 increases, the acid strength increases best describes the relationship between acid strength and Ka value for weak acids.
increases, the acid strength increases best describes the relationship between acid strength and Ka value for weak acids.