Answer: Option (d) is the correct answer.
Step-by-step explanation:
Kinetic energy is the energy of a substance due to its motion. So, when we increase the temperature of substance then there will be increase in motion of particles.
Therefore, they will gain kinetic energy with increase in temperature.
Also, it is known that kinetic energy is directly proportional to temperature through the relation as follows.
K.E =
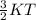
Thus, more is the temperature more will be kinetic energy of a substance. Hence, we can conclude that the temperature of the substance is the factor which is defined as the average kinetic energy of a substance.