Answer:
Mn is the oxidizing agent.
N is the reducing agent.
Step-by-step explanation:
Hello!
In this case, according to the undergoing chemical reaction, it is seen that the manganese in KMnO4 has an oxidation state of 7+, in MnSO4 of 2+ and nitrogen in KNO2 is 3+ and in KNO3 is 5+; thus we have the following half-reactions:
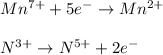
Thus, since manganese is undergoing a decrease in the oxidation state, we infer it is the oxidizing agent whereas nitrogen, undergoing an increase in the oxidation state is the reducing agent.
Best regards!