Answer: The correct answer is 4 moles.
Step-by-step explanation:
For the given chemical reaction:
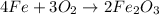
Oxygen from air is available which means it is present in excess. Iron is considered as a limiting reagent because it limits the formation of products.
By Stoichiometry of the reaction:
4 moles of iron produces 2 moles of iron oxide.
So, 8 moles of iron will produce =
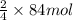 of iron oxide.
of iron oxide.
Hence, the correct answer is 4 moles.