Answer:
Option A
Step-by-step explanation:
Octet rule applies to element and it says that in order to achieve noble gas configuration, the outermost shell (valence shell) must have eight electron.
Given
Number of electrons in fluorine in its outermost shell
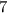
Number of electrons to fulfill the octet rule
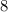
Deficiency of electron
 Number of electrons to fulfill the octet rule
Number of electrons to fulfill the octet rule
 Number of electrons in fluorine in its outermost shell
Number of electrons in fluorine in its outermost shell
Substituting the given values in above equation, we get
Deficiency of electron
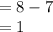
Hence, option A is correct.