Answer 1: The percentage error associated with Jason's measurement is 0.6%.
Step-by-step explanation:
Experimental value of density if iron = 7.921 g/mL
Actual value of density of iron = 7.874 g/mL
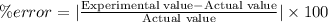
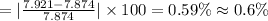
Hence, the correct an option is (C).
Answer 2: The correct answer is bar graph.
Step-by-step explanation:
In order to express the solubility of three different salt in water at 22 ° C graphically Rob must use bar graph. By keeping the solubility on vertical axis and and bars of different salt on horizontal axis with their heights respective their solubility values. Hence, the correct answer is option (A).
As shown in an image attached.