Answer:

Step-by-step explanation:
To convert from moles to grams, we must use the molar mass. This can be found on the Periodic Table.
We are given a sample of sulfur. Look for the symbol S on the table.
This tells us the mass of 1 mole of sulfur. Use this molar mass as a ratio.
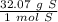
Multiply by the given number of grams (707).
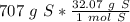
Flip the fraction so the grams of sulfur will cancel out.
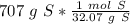
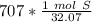
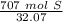
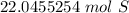
The number we caluclated is the closest to answer choice D: 22.1 moles of sulfur