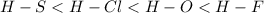Answer: The correct answer is
Step-by-step explanation:
Polarity of the bond is determined with the help of electronegativity difference of the elements which form a bond. More the electronegativity difference, bond will be more polar and vice-versa.
We are given the electronegativity values of the following elements:
Electronegativity of Hydrogen = 2.2
Electronegativity of Fluorine = 4.0
Electronegativity of Chlorine = 3.16
Electronegativity of Oxygen = 3.44
Electronegativity of Sulfur = 2.58
- For
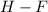
Electronegativity difference = 4.0 - 2.2 = 1.88
- For
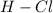
Electronegativity difference = 3.16 - 2.2 = 0.96
- For

Electronegativity difference = 3.44 - 2.2 = 1.24
- For
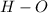
Electronegativity difference = 2.58 - 2.2 = 0.38
As, from the calculations above, it is visible that the electronegativity difference of HF bond is more, and it is least for HS bond. Hence, the increasing order of bond polarity will be: