Answer: 971.428 kg of ammonia will react with 2800 kg of phosphoric acid.
Step-by-step explanation:
Mass of sodium hydrogen carbonate in 1 tablet =2800 kg = 2,800,000 g
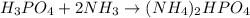
Moles of
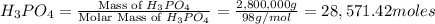
According to reaction 1 mole of
![]H_3PO_4](https://img.qammunity.org/2018/formulas/chemistry/high-school/l6jxg0qf9cmn2b9ibkm2b8genkx1ygr4o1.png) reacts with 2 mole of
reacts with 2 mole of
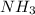 then 28,571.42 mole of
then 28,571.42 mole of
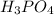 will react with:
will react with:
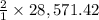 moles of
moles of
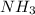 that is 57,142.84 moles.
that is 57,142.84 moles.
Mass of
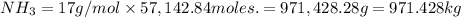
971.428 kg of ammonia will react with 2800 kg of phosphoric acid.