Answer: (2)
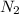
Explanation:
1)
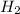 is a diatomic molecule which is formed by sharing of two electrons between two hydrogen atoms.
is a diatomic molecule which is formed by sharing of two electrons between two hydrogen atoms.
2)
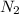 is a diatomic molecule which is formed by sharing of six electrons between two nitrogen atoms.
is a diatomic molecule which is formed by sharing of six electrons between two nitrogen atoms.
3)
 is a diatomic molecule which is formed by sharing of four electrons between two oxygen atoms.
is a diatomic molecule which is formed by sharing of four electrons between two oxygen atoms.
4)
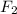 is a diatomic molecule which is formed by sharing of two electrons between two fluorine atoms.
is a diatomic molecule which is formed by sharing of two electrons between two fluorine atoms.