The chemical reaction is given as:
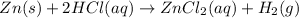
First, calculate the number of moles of zinc:
Number of moles =
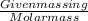
Given mass of zinc =
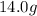 and molar mass of zinc =
and molar mass of zinc =
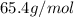
Number of moles =
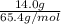
=
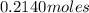
Now, moles of hydrogen =
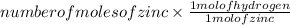 ( as 1 mole of zinc gives 1 mole of hydrogen)
( as 1 mole of zinc gives 1 mole of hydrogen)
=
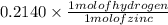
=
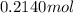 of hydrogen.
of hydrogen.
Volume of hydrogen is calculated by:
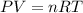
where, P = pressure = 1 atm at STP
V = volume
n= number of moles
R = gas constant =
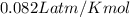
T = temperature= 273 K at STP
Now, insert the values in formula, we get
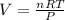
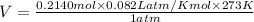
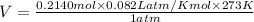
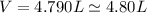
Thus, volume of hydrogen is
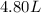 i.e. second option is the correct answer.
i.e. second option is the correct answer.