Answer: The pressure exerted by hydrogen gas is 0.33 atm
Step-by-step explanation:
To calculate the number of moles, we use the equation:
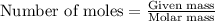
Given mass of zinc = 2.57 g
Molar mass of zinc = 65.4 g/mol
Putting values in above equation, we get:
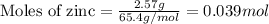
We are given:
Moles of HCl = 0.500 moles
For the given chemical equation:
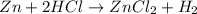
By Stoichiometry of the reaction:
1 mole of zinc reacts with 2 moles of HCl
So, 0.039 moles of zinc will react with =
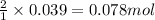 of HCl
of HCl
As, given amount of HCl is more than the required amount. So, it is considered as an excess reagent.
Thus, zinc metal is considered as a limiting reagent because it limits the formation of product.
By Stoichiometry of the reaction:
1 mole of zinc reacts with 1 mole of Hydrogen gas
So, 0.039 moles of zinc will react with =
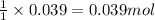 of hydrogen gas
of hydrogen gas
To calculate the pressure of hydrogen gas, we use the equation given by ideal gas:
PV = nRT
where,
P = Pressure of hydrogen gas = ?
V = Volume = 3.00 L
n = number of moles of hydrogen gas = 0.039 moles
R = Gas constant =
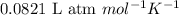
T = Temperature =
![35.8^oC=[35.8+273]K=308.8K](https://img.qammunity.org/2019/formulas/chemistry/college/zgaez2dapkkjeghdsr8iqlc74lcs5hrc0c.png)
Putting values in above equation, we get:
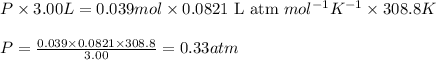
Hence, the pressure exerted by hydrogen gas is 0.33 atm