Answer : The fourth quantum number for one of the electrons in the 4p energy sublevel of bromine is,

Explanation :
There are 4 quantum numbers :
Principle Quantum Numbers : It describes the size of the orbital. It is represented by n. n = 1,2,3,4....
Azimuthal Quantum Number : It describes the shape of the orbital. It is represented as 'l'. The value of l ranges from 0 to (n-1). For l = 0,1,2,3... the orbitals are s, p, d, f...
Magnetic Quantum Number : It describes the orientation of the orbitals. It is represented as
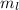 . The value of this quantum number ranges from
. The value of this quantum number ranges from
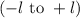 . When l = 2, the value of
. When l = 2, the value of
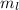 will be -2, -1, 0, +1, +2.
will be -2, -1, 0, +1, +2.
Spin Quantum number : It describes the direction of electron spin. This is represented as
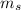 The value of this is
The value of this is
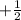 for upward spin and
for upward spin and
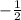 for downward spin.
for downward spin.
In the bromine atom the one electron in 4p energy sublevel,
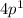 .
.
Now we have to calculate the values of all the quantum numbers.
For
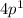 :
:
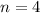 (Because it is in 4rd shell)
(Because it is in 4rd shell)
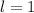 (Because it is in 'p' orbital)
(Because it is in 'p' orbital)
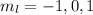 (Because l = 1)
(Because l = 1)
 (Because of the sign convention)
(Because of the sign convention)
Therefore, the fourth quantum number for one of the electrons in the 4p energy sublevel of bromine is,
