Hello!
1) What is the limiting reactant?The chemical equation for this reaction is:
2Al(OH)₃ → Al₂O₃ + 3H₂OThe
Limiting Reactant is the one that is in the lowest amount in the reaction and determines how many moles are produced. You can see that in this chemical equation there is only one reactant since this reaction is a
decomposition reaction. So,
the limiting reactant is Al(OH)₃.2) How many grams of water are produced?The chemical equation for this reaction is:
2Al(OH)₃ → Al₂O₃ + 3H₂O
So, we are going to use the following conversion factor to go from grams of Al(OH)₃ to grams of water:
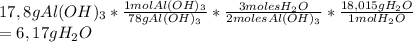
So,
6,17 grams of Water are produced.
Have a nice day!