Answer:
KI is not expected to be soluble in cyclohexane
Step-by-step explanation:
- KI is an ionic solid. In crystal of KI,
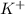 and
and
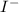 ions are held together through coloumbic attraction force.
ions are held together through coloumbic attraction force. - Cyclohexane is a non-polar solvent. Only van der waal force exists between cyclohexane molecules.
- During dissolution of KI in cyclohexane, cyclohexane molecules exerts only van der waal force to KI molecules.
- But this weak van der waal force cannot overcome the coloumbic attraction force exists between KI molecules during dissolution.
- Hence KI is not expected to be soluble in cyclohexane.