Hello!
The chemical equation for this reaction is the following:
2HCl (aq) + Ca(OH)₂(aq) → CaCl₂(aq) + H₂O(l)
To determine the grams of Calcium Chloride formed, we will need to determine the moles of each reactant and divide by the reaction coefficient to know which is the limiting reactant (the one that is in the lowest amount):
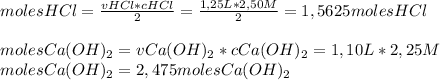
So, the limiting reactant is HCl, so we'll use the following conversion factor to determine the mass of CaCl₂ produced:
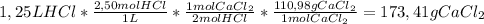
So,
173,41 grams of CaCl₂ are produced.
Have a nice day!