Answer:- None of the choice is correct. The enthalpy for the combustion of 1 mol of pentaborane is -4543.05 kJ and so none of the choice is correct.
Solution:- First of all we write the balanced equation for the combustion of one mol of pentaborane:
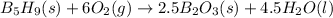
![\Delta H_r_x_n^0=[\sum \Delta H_f]_p_r_o_d_u_c_t-[\sum \Delta H_f]_r_e_a_c_t_a_n_t](https://img.qammunity.org/2019/formulas/chemistry/college/12m92qa4jg1s6objkfm8rspo4bnipoa4a4.png)
Let's plug in the given values in the formula:
![\Delta H_r_x_n^0=[2.5(-1273.5)+4.5(-285.8)]-[73.2+6(0)]](https://img.qammunity.org/2019/formulas/chemistry/college/vkw5kxjz11fcmvnmd0epungy2omy0djbaq.png)
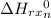 = -3183.75 - 1286.1 - 73.2
= -3183.75 - 1286.1 - 73.2
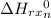 = -4543.05 kJ
= -4543.05 kJ
From the above calculations, the enthalpy for the combustion of 1 mol of pentaborane is -4543.05 kJ and so none of the choice is correct.