Answer:
For NaCl: The correct answer is Option A.
For
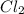 : The correct answer is Option B.
: The correct answer is Option B.
Step-by-step explanation:
Molar mass is defined as the sum of the mass of all the atoms each multiplied its atomic masses that are present in the molecular formula of a compound. It is expressed in g/mol.
For the given chemical reaction:
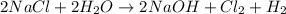
The reactants are
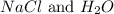
The products are
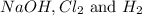
We know that:
Molar mass of Sodium = 22.99 g/mol
Molar mass of Chlorine = 35.45 g/mol
For starting substance NaCl:
Molar mass of NaCl =
![[(1* 22.99)+(1* 35.45)]=58.44g/mol](https://img.qammunity.org/2019/formulas/chemistry/college/63k4mjg4gvk9lsn9jbutrqkc01pis1ytfe.png)
Hence, the correct answer is Option A.
For starting substance
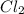 :
:
Molar mass of
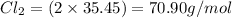
Hence, the correct answer is Option B.