Answer:
The partial pressure of the oxygen gas in the mixture is 80 mmHg.
Step-by-step explanation:
Total pressure of the gas mixture ,p = 800 mmHg
Percentage of oxygen gas = 10% = 0.10 =

Percentage of nitrogen gas = 90% = 0.90=
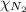
Partial pressure an individual gas in a mixture is given by Dalton's law of partial pressure:
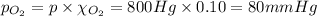
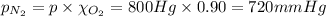
The partial pressure of the oxygen gas in the mixture is 80 mmHg.