Answer: The specific heat will be
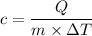
Step-by-step explanation:
Given that,
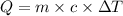
Here,
Q = Energy
m = molar mass
c = specific heat
 = Temperature
= Temperature
The specific heat will be
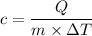
The specific heat defined as the ratio of heat required per unit mass and per unit change in temperature.
Hence, The specific heat will be
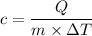 .
.