Hello!
The overall
photosynthesis reaction is the following one:
6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂
To calculate how many moles of H₂O are needed it a plant produces 4,76 moles of C₆H₁₂O₆ (glucose) we'll need to use the following conversion factor, applying the reaction coefficients from the chemical equation of the photosynthesis reaction:
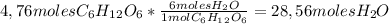
So,
28,56 moles of H₂O are required by a plant to produce 4,76 moles of Glucose, in the Photosynthesis reaction.
Have a nice day!