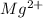Answer: The magnesium ion formed is
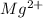
Step-by-step explanation:
Magnesium is the 12th element of the periodic table having electronic configuration:
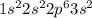 . This element will easily loose 2 electrons and form
. This element will easily loose 2 electrons and form
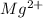 ion.
ion.
Sulfur is the 16th element of the periodic table having electronic configuration:
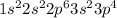 . This element will easily gain 2 electrons and form
. This element will easily gain 2 electrons and form
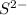 ion.
ion.
Sulfur and magnesium will form ionic compound, which means that a complete transfer of electrons takes place from one element to another.The compound formed will be MgS (Magnesium sulfide).
Hence, the magnesium ion formed is