Answer : This chemical equation represents a single replacement reaction.
Explanation :
Single replacement reaction : It is defined as the reaction in which the most reactive metal displace the least reactive metal form its solution.
The given balanced chemical reaction is:
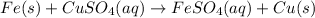
The given chemical reaction is a single replacement reaction because in this reaction, iron is most reactive metal than the copper. So, iron can displace the copper metal form its solution and it forms iron (II) sulfate and copper as a product.
Hence, the chemical equation represents a single replacement reaction.